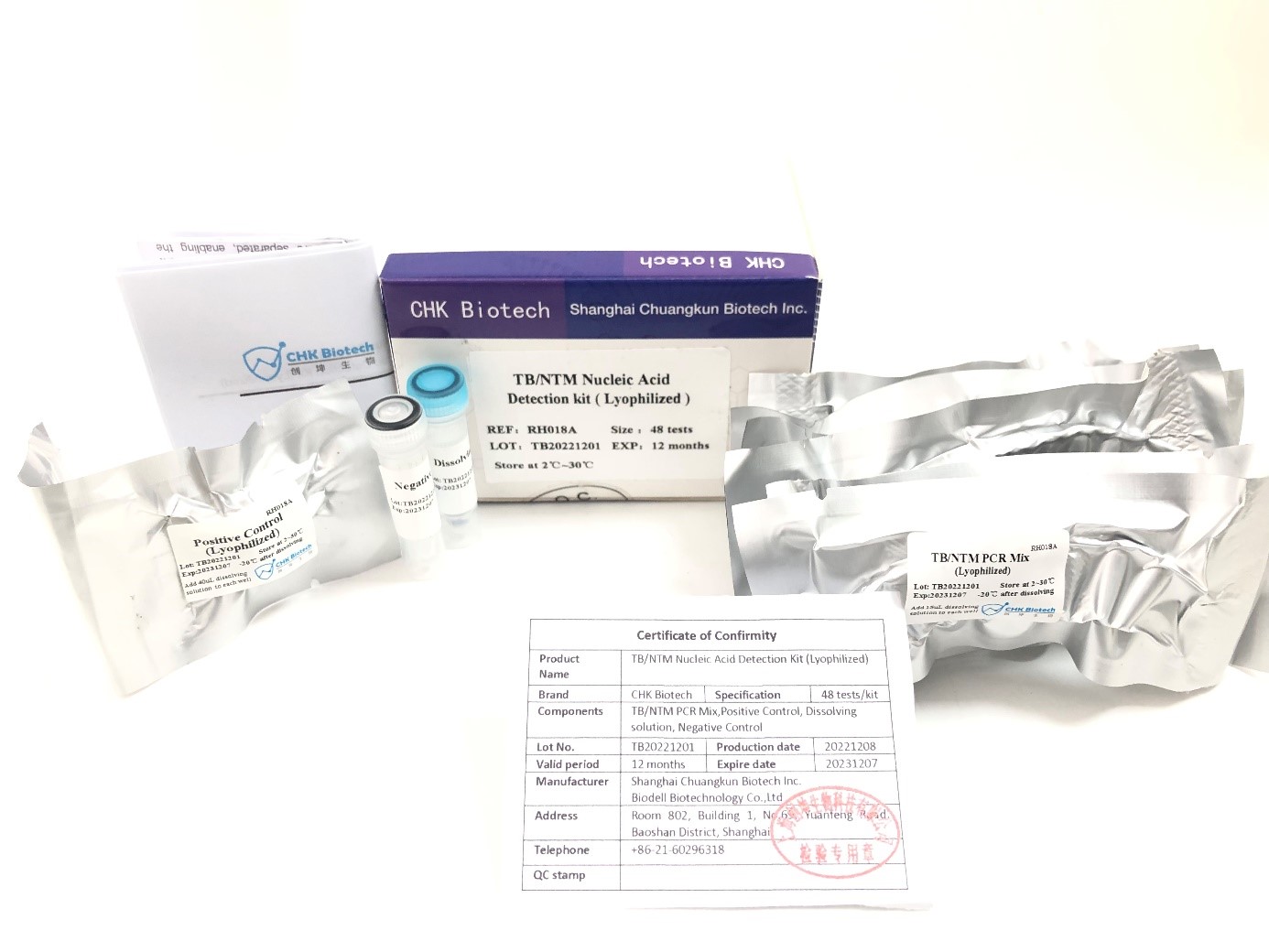
On the 1st day, Februray, Shanghai Chuangkun Biotechnology Co., Ltd. obtained the second registration certificate of Thailand FDA for TB/NTM-DNA detection kit, steped by the 15 type HPV detection PCR kit one month ago. which declared that Chuangkun Biotech’s products have been authorized by Thailand FDA, improving self-developed products,and also providing strong support for Chuangkun Biotech to further expand the international market.
An estimated global total of 10.6 million people fell ill with TB published by WHO in 2021.The increasing rate by 4.5% more than the year 2020.Meanwhile 1.6 million people died of tuberculosis last year(including 6.7% were among people living with HIV). Geographically, most TB cases in 2021 were in the WHO regions of South-East Asia (45%), Africa (23%) and the Western Pacific (18%), with smaller shares in the Eastern Mediterranean (8.1%), the Americas (2.9%) and Europe (2.2%). Considering the COVID-19 Pandemic,tuberculosis prevention and controlling is more and more harshed for the Plan of Termination of Tuberculosis for WHO.
TB/NTM-DNA detection kit for CHUANGKUN Biotech is creatived by lyophilization procedure The process will solve the transportation long-term disadvantages for the traditional PCR detection kit, also brings excellent accuracy and high specificity results more over covenience for uses.
Shanghai Chuangkun Biotechnology Co., Ltd. obtained the second registration certificate of Thailand FDA for TB/NTM-DNA detection kit,indicates recognition by the Thailand FDA. Chuangkun Biotech will parctise in the regional tuberculosis prevention and controlling vigorously.
Post time: Feb-02-2023

 中文
中文